
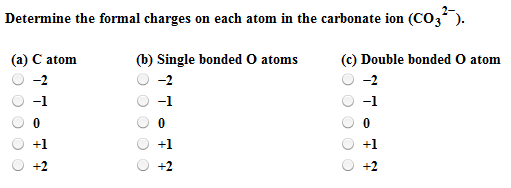
By doing so, you will get the following lewis structure of CO 3 2- ion. In the above lewis dot structure of CO 3 2- ion, you can also represent each bonding electron pair (:) as a single bond (|). This overall -2 charge on the CO 3 molecule is represented in the image given below. So let’s keep these charges on the respective atoms in the CO 3 molecule. You can see the number of bonding electrons and nonbonding electrons for each atom of CO 3 2- molecule in the image given below.įor Carbon (C) atom: Valence electron = 4 (because carbon is in group 14) Bonding electrons = 8 Nonbonding electrons = 0įor single bonded Oxygen (O) atom: Valence electrons = 6 (because oxygen is in group 16) Bonding electrons = 2 Nonbonding electrons = 6įor double bonded Oxygen (O) atom: Valence electrons = 6 (because oxygen is in group 16) Bonding electrons = 4 Nonbonding electrons = 4 Formal chargeįrom the above calculations of formal charge, you can see that the single bonded oxygen (O) atom has -1 charges and the other atoms have 0 charges. In short, now you have to find the formal charge on carbon (C) atom as well as oxygen (O) atoms present in the CO 3 2- ion.įor calculating the formal charge, you have to use the following formula įormal charge = Valence electrons – (Bonding electrons)/2 – Nonbonding electrons The stability of lewis structure can be checked by using a concept of formal charge. Now you have come to the final step in which you have to check the stability of lewis structure of CO 3 2- ion. Step 6: Check the stability of lewis structure Total valence electrons in CO 3 2- ion = valence electrons given by 1 carbon atom + valence electrons given by 3 oxygen atoms + 2 more electrons are added due to 2 negative charges = 4 + 6(3) + 2 = 24. The formal charge on three oxygen atom has electron pair shared by chlorine. You can see the 6 valence electrons present in the oxygen atom as shown in the above image. Click hereto get an answer to your question Calculate formal charge of atoms HClO4, CO32. sodium ions exactly cancel the single -2 charge from the carbonate ion, and the. Hence the valence electrons present in oxygen is 6. The non-metals carbon (C) and silicon (Si) generally dont form cations. Oxygen is group 16 element on the periodic table. → Valence electrons given by oxygen atom: You can see the 4 valence electrons present in the carbon atom as shown in the above image. Hence the valence electrons present in carbon is 4.

→ Valence electrons given by carbon atom:Ĭarbon is group 14 element on the periodic table.
Here, I’ll tell you how you can easily find the valence electrons of carbon as well as oxygen using a periodic table. (Valence electrons are the electrons that are present in the outermost orbit of any atom.) In order to find the total valence electrons in CO3 2- ion, first of all you should know the valence electrons present in the carbon atom as well as oxygen atom. Steps of drawing CO3 2- lewis structure Step 1: Find the total valence electrons in CO3 2- ion


 0 kommentar(er)
0 kommentar(er)
